Is Enthalpy A State Function
The enthalpy for the state C can be picked directly from steam tables whereas the enthalpy for the state D must be calculated using vapor quality. As such it is one of the four fundamental states of matter the others being solid gas and plasma and is the only state with a definite volume but no fixed shapeA liquid is made up of tiny vibrating particles of.
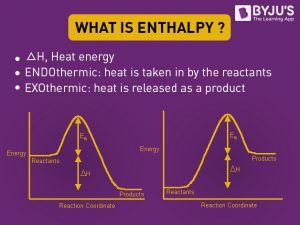
What Is Enthalpy Definition Endothermic Exothermic Reaction
Hesss law is now understood as an expression of the fact that the enthalpy of a chemical process is independent of the path taken from the initial to the final state ie.

. Web Just as with ΔU because enthalpy is a state function the magnitude of ΔH depends on only the initial and final states of the system not on the path taken. Web Enthalpy is also a state function. Web The Physics Classroom serves students teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional.
To find ΔH for a reaction measure q_p. Chemistry also addresses the nature. For reactions which are incomplete the equilibrium constant can be determined as a function of temperature.
Web The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken. Web In chemistry and thermodynamics the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is. The heat given off or absorbed when a reaction is run at constant pressure is equal to the change in the enthalpy of the system.
Josiah Willard Gibbs in his papers used the term fundamental functions. The transition comprises a smooth increase in the viscosity of a material by as much as 17 orders of magnitude within a temperature range of 500 K without any pronounced change in material structure. Fruits consist of ethylene.
If it is in the liquid state need extra energy to convert it from liquid state to gaseous state. One main thermodynamic potential. Web The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system.
The intellectual property rights and the responsibility for accuracy reside wholly with the author Dr. There is an increase in ethylene production when fruit gets damaged or when it is plucked. The reversible heat is the enthalpy change for the transition and the entropy change is the enthalpy change divided by.
Enthalpy is an energy-like property or state functionit has the dimensions of energy and is thus measured in units of joules or ergs and its value is determined entirely by the temperature pressure and composition of the system and not. Consider an example of hydrogenation of propene which has the following. Web A thermodynamic potential or more accurately a thermodynamic potential energy is a scalar quantity used to represent the thermodynamic state of a systemThe concept of thermodynamic potentials was introduced by Pierre Duhem in 1886.
The change in the enthalpy of the system during a chemical reaction is equal to the change in the internal energy plus the change in the product of the pressure of the gas in the system and its volume. The equation for the standard enthalpy change of formation originating from Enthalpys being a State Function shown below is. Enthalpy is a state function.
It is a natural science that covers the elements that make up matter to the compounds made of atoms molecules and ions. Enthalpy values for specific substances cannot be measured directly. Web Since enthalpy is a state function its value is the same for any path between given initial and final states so that the measured ΔH is the same as if the temperature stayed constant during the combustion.
HyperPhysics is provided free of charge for all classes in the Department of Physics and Astronomy through internal networks. Gas liquid solid crystal or. Web The server for HyperPhysics is located at Georgia State University and makes use of the Universitys network.
Their composition structure properties behavior and the changes they undergo during a reaction with other substances. Web Chemistry is the scientific study of the properties and behavior of matter. H sys q p.
For processes that take place at constant pressure a common condition for many chemical and physical changes the enthalpy change Δ H is. Written by teachers for teachers and students The Physics Classroom provides a wealth of resources that meets the varied needs of both. Hank feels bad for our friend and wants us to learn more about.
Web The state function was called the internal energy that is central to the first law of thermodynamics. Web enthalpy the sum of the internal energy and the product of the pressure and volume of a thermodynamic system. Web The use of statistical mechanics and the partition function is an important tool throughout all of physical chemistry because it is the key to connection between the microscopic states of a system and the macroscopic variables which we can measure such as temperature pressure heat capacity internal energy enthalpy and entropy just to.
O A degree signifies that its a standard enthalpy change. The glass transition of a liquid to a solid-like state may occur with either cooling or compression. Bond enthalpy can be calculated directly if everything your working is in a gaseous state.
The consequence of this dramatic increase. Web A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. Web The symbol of the standard enthalpy of formation is ΔH f.
Web Energy is like the bestest best friend ever and yet most of the time we take it for granted. Using the state postulate take the specific entropy for a homogeneous substance to be a function of specific volume and temperature. F The f indicates that the substance is formed from its elements.
Most important the enthalpy change is the same even if the process does not occur at constant pressure. Latent heat of vaporization water at 01 MPa atmospheric pressure h lg 2257. It involves a series of changes.
Only enthalpy changes for chemical or physical processes can be determined. The ClausiusClapeyron relation characterizes behavior of a closed system during a phase change at constant temperature and pressureTherefore. 508.
Δ A change in enthalpy. BYJUS is Indias largest ed-tech company and the creator of Indias most loved school learning app. G H - TS.
Bond enthalpy to estimate the enthalpy of the reaction. Web Derivation from state postulate. Web The enthalpy of vaporization is a function of the pressure at which that transformation takes place.
Launched in 2015 BYJUS offers highly personalised and effective learning programs for classes 1 - 12 K-12 and aspirants. Web In the thermodynamics of equilibrium a state function function of state or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities that describe equilibrium states of a system that depend only on the current equilibrium thermodynamic state of the system eg. The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions.
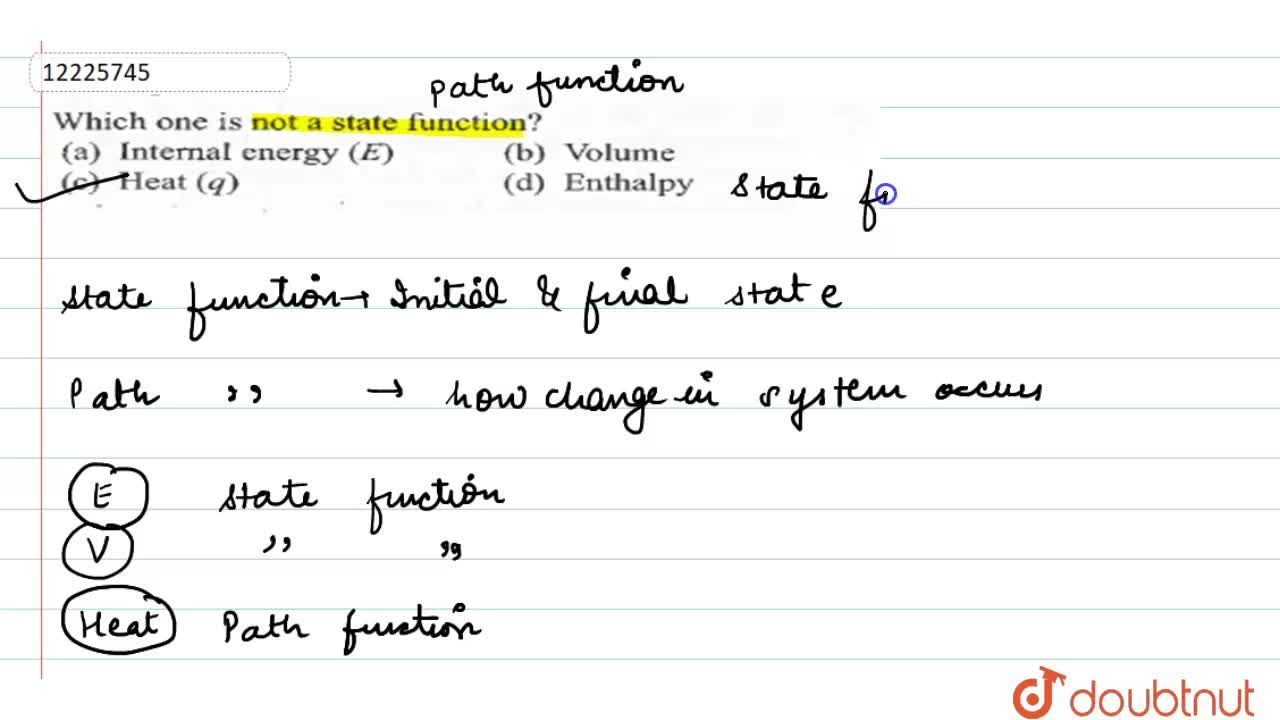
Which One Is Not A State Function

State Function Property With A Specific Value Only Influenced By A System S Present Condition Only Dependent On The Initial And Final States Ppt Download

Identify The State Functions From The Following

Chem101 9 14 Enthalpy Is A State Function Youtube

Enthalpy The Meaning Of Enthalpy 1 Enthalpy Is A State Function With The Symbol H H E Pv E Is The Internal Energy Of The System P Is The Pressure Ppt Download
Chapter 2 First Law The Concepts

Change In Enthalpy State Function Dh Q At Constant Pressure Ppt Download

State Functions Infinity Learn
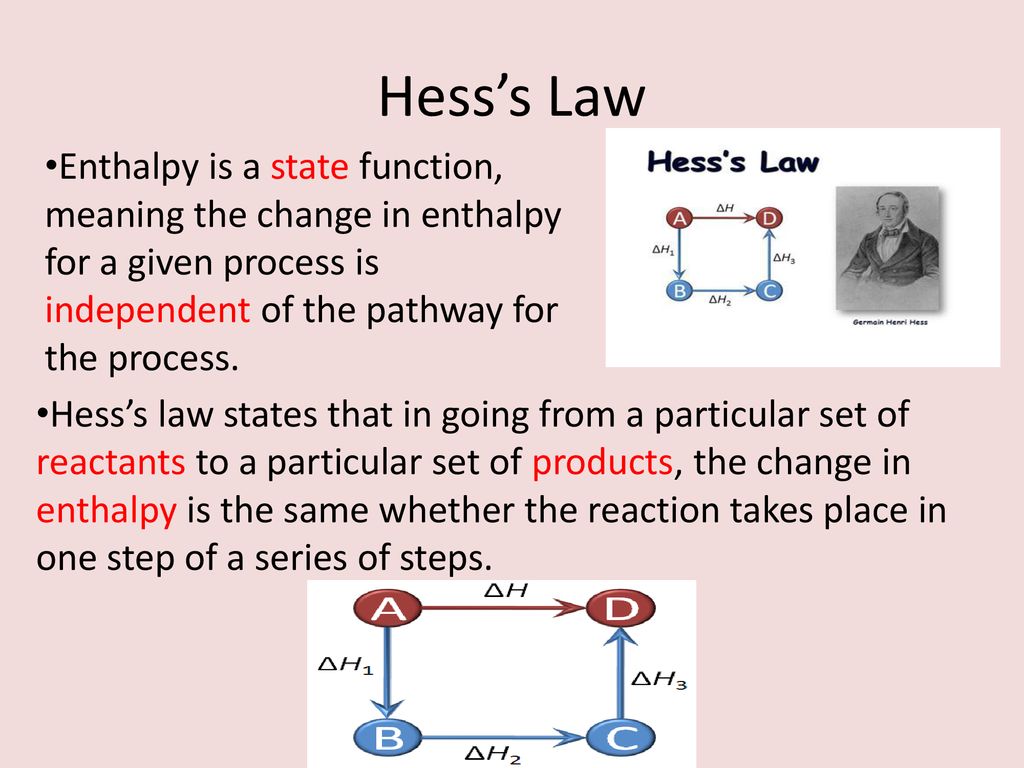
Energy And Chemical Reactions Ppt Download

Which One Of The Following Statements About State Functions Is Correct A Internal Energy Enthalpy Heat And Work Are All Thermodynamic State Functions B A State Function Depends Both On The Past
Helmholtz And Gibbs Free Energies
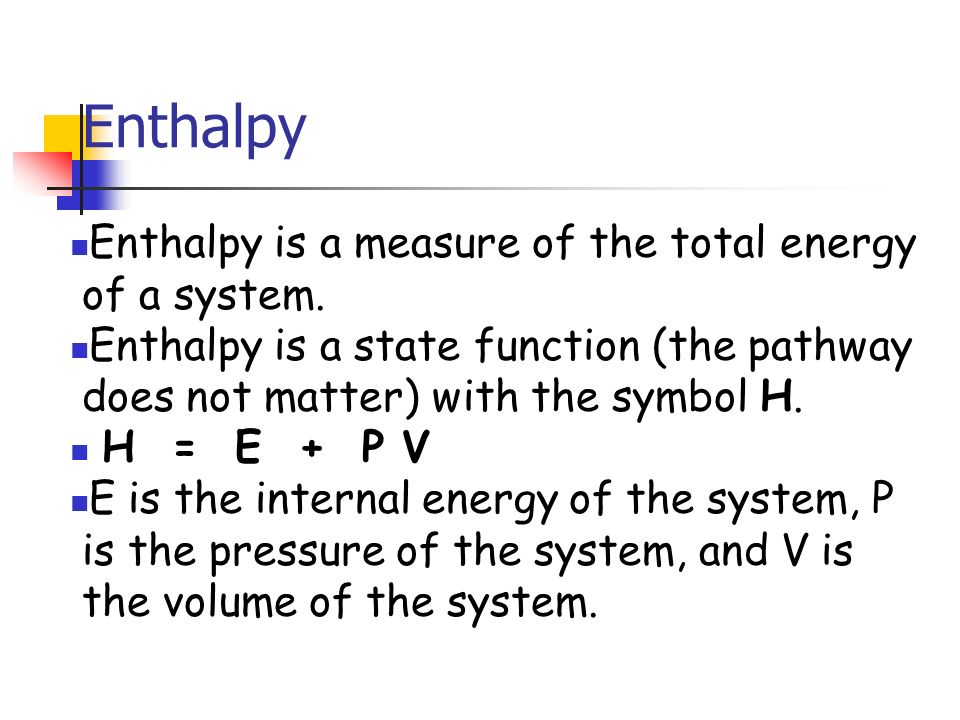
Enthalpy Enthalpy Is A Measure Of The Total Energy Of A System Enthalpy Is A State Function The Pathway Does Not Matter With The Symbol H H E P Ppt Download

State Functions And Enthalpy Chapter 5 Part 6 Youtube

State Functions Thermodyanmics Concepts Explanation Embibe

Enthalpy Really Is A State Function Hess S Law Chem 152 Study Notes Chemistry Docsity

Explain State Function Enthalpy Brainly In

Thermodynamics Part 1 Work Heat Internal Energy And Enthalpy